Everyday Food Safety
Food Traceability System: A Step-by-Step Guide for Safer Food Supply Chain
Food traceability is not just a management tool; it’s a vital component for ensuring food safety and compliance throughout the supply chain. By actively tracking and uniquely identifying product units, you gain valuable insights into the history and location of your products.
Here’s how to effectively design and implement a robust food traceability system.
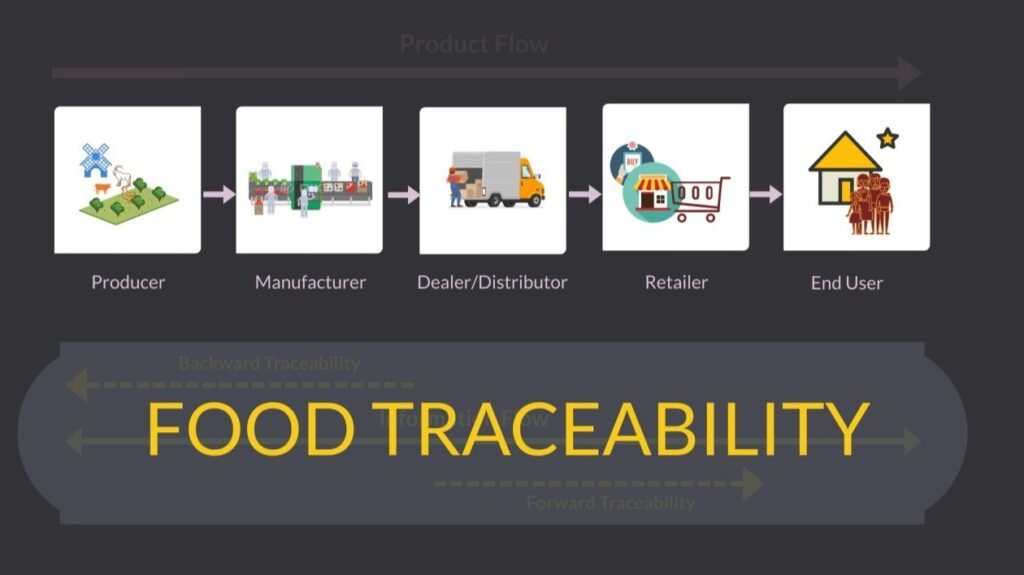
What are the Steps for Designing a Food Traceability System?
- Identify Your Role. First, determine your position within the feed and food supply chain. Are you a supplier, manufacturer, distributor, or retailer? Understanding your role will help you manage the traceability process effectively.
- Analyze Material Flow. Next, track the movement of materials from incoming supplies to outgoing finished products. This step is essential for understanding your operational scope and ensuring a smooth traceability process.
- Define Information Requirements. Your role in the supply chain determines the specific information necessary to develop your traceability system.
What are the 3 Key Components of a Food Traceability System?
- Supplier or Backward Traceability. This involves identifying and tracking the products you receive. Document all relevant information from the source, including:
- Description of the supplied product
- Delivery date and quantity of the product
- Analysis and testing records
- Batch code or lot number
- Hauler/truck details used for transportation
- Supplier name, address, and contact details
- Process or Internal Traceability. Track and record all steps in the production process, from receipt of incoming materials to the completion of finished products. Essential information includes:
- Product description and unique identification code
- Production details such as shift, date, and time
- Quantity produced and documentation for ingredients
- In-process analysis and testing records
- Customer or Forward Traceability. Finally, track the destination of your finished products. Make sure you have the documentation on the following:
- Product description and batch code
- Dispatch and receiving records
- Customer and FBO details
- Distribution date and sales receipt
- QA/QC release records
How to Establish Your Traceability System?
To establish an effective traceability system, follow these essential steps:
- Develop a Traceability Plan. Develop a comprehensive plan that covers all identified requirements.
- Assign Responsibilities. Establish a traceability team with clearly defined roles and responsibilities. Ideally, this team should comprise five members, including some HACCP team members.
- Develop a Training Plan. Ensure that all relevant personnel receive proper training to implement the traceability plan effectively.
- Monitor and Review: Regularly review your traceability system at least once a year. Perform internal audits to evaluate effectiveness and make necessary improvements.
What are the benefits of a strong traceability system?
- Enhances reliability and compliance preparedness.
- Reduces the risk of costly recalls and fines.
- Fosters accountability and a food safety culture.
Conclusion
In conclusion, implementing a food traceability system is essential for enhancing food safety, maintaining regulatory compliance, and fostering consumer trust. By systematically tracking each step in the supply chain—from sourcing to final delivery—you not only minimize the risks of recalls and product mismanagement, but also position your business for long-term success. Whether you are just starting your traceability journey or looking to strengthen an existing system, committing to robust traceability practices is a proactive investment in the safety, quality, and reputation of your food supply chain.
Key Takeaways
- Food Traceability System ensures safety and compliance by tracking product units throughout the supply chain.
- Key steps for designing a system include identifying your role, analyzing material flow, and defining information requirements.
- The three key components are supplier, internal, and customer traceability.
- Establish your system by developing a comprehensive traceability plan, assigning responsibilities, and providing proper training.
- A strong traceability system enhances reliability, reduces recall risks, and fosters a food safety culture.
Ready to Improve Your Food Traceability?
Implementing a robust traceability process is essential for ensuring food safety and compliance. Whether you’re preparing for regulatory inspections or auditing your supply chain, having the right tools can make a significant difference.
Here’s our comprehensive Food Traceability Audit Checklist, which streamlines audits, identifies gaps, and helps you stay prepared.




